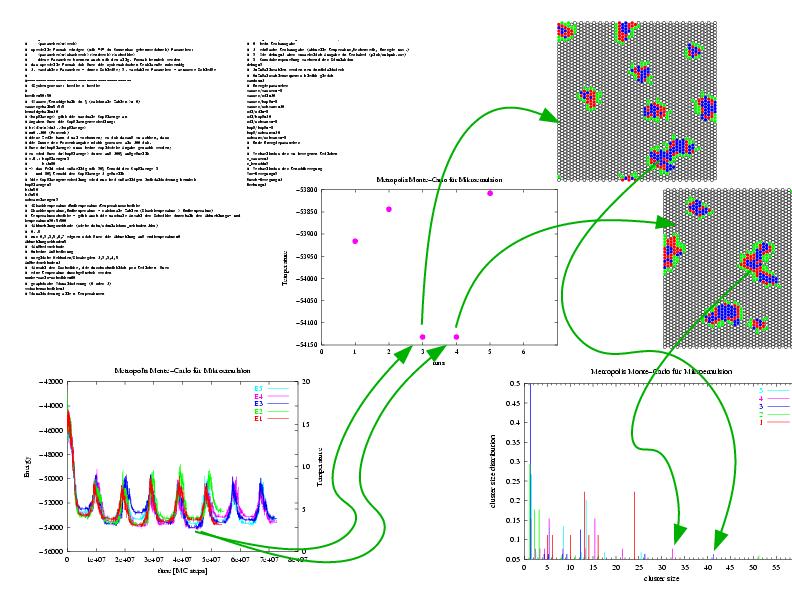
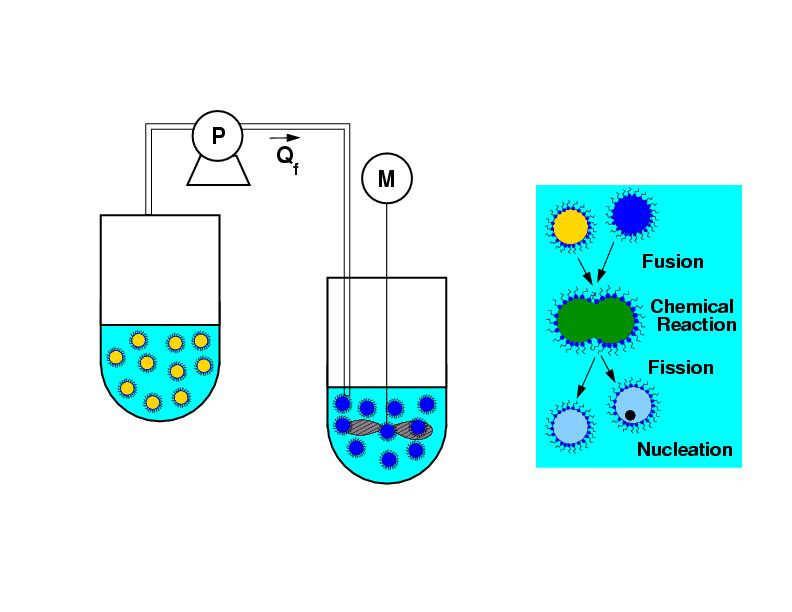
 Particle precipitation in microemulsion droplets:
The exchange, reaction, nucleation and growth phenomena and their interplay on different time scales
are the important processes to model the particle precipitaion in microemulsion.
A kinetic Monte-Carlo method is applied to this problem. It is used to investigate the dynamics of particle
precipitation as well as to indentify suitable process control parameters for an upscale approach
of this technology.
Particle precipitation in microemulsion droplets:
The exchange, reaction, nucleation and growth phenomena and their interplay on different time scales
are the important processes to model the particle precipitaion in microemulsion.
A kinetic Monte-Carlo method is applied to this problem. It is used to investigate the dynamics of particle
precipitation as well as to indentify suitable process control parameters for an upscale approach
of this technology.
| Home | Disclaimer | Process Systems Engineering Magdeburg | University Magdeburg | Center for Simulational Physics UGA | Dep. of Physics and Astronomy UGA | University of Georgia Athens |